Introduction
In the context of the continuous development of the global pharmaceutical industry, drug safety and production efficiency have become the core goals pursued by various pharmaceutical companies. In recent years, with the gradual updating and improvement of the appendix of the “Good Manufacturing Practice for Drugs” (2010 version GMP) regulations, the country’s supervision and requirements for the pharmaceutical industry have become increasingly strict.
In the face of these challenges, Canaan Technology is no longer satisfied with assisting customers in providing verification documents. It can now provide full process verification from device installation to performance qualification (PQ), with comprehensive service upgrades.
Verify Service Cases
Taking a pharmaceutical company in Tianjin as an example, the customer has newly purchased a Coater with Perforated Drum and a Post Bin Blender, and Canaan Technology is responsible for the design, production, installation, commissioning, testing, and verification work.
After the equipment arrives at the workshop, both parties jointly determine the verification plan and conduct equipment verification.


▲ Equipment Display Diagram
Canaan Verification Service Process
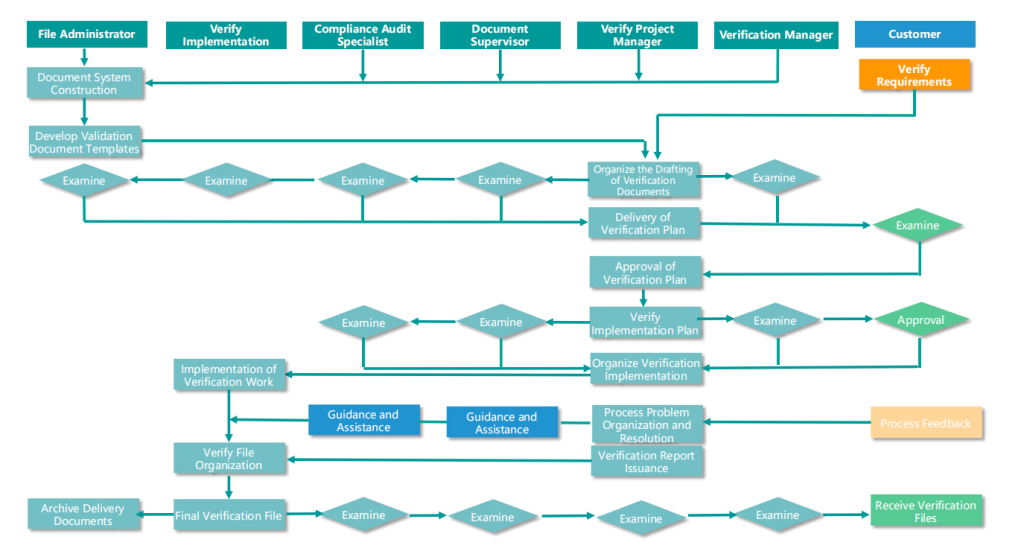
▲ Verification Service Flow Chart
According to the flowchart, Canaan Technology provides the following verification services to customers:
Phase 1: User Requirements URS
Canaan Technology has had in-depth communication with customers to ensure that the equipment validation plan, execution, reporting, rectification, and deviation handling can meet GMP requirements. The equipment validation engineers, based on their own experience, complete the validation work on time and with quality within the planned time, and promptly follow up and handle deviations or changes.
Phase 2: System Risk Assessment RA
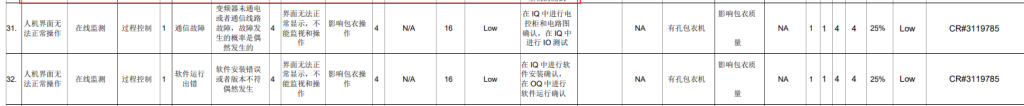
▲ Project Information Diagram
Phase 3: Confirmation/Verification Activities
The validation life-cycle of the equipment mainly adopts the equipment validation process proposed in the 2023 GMP-API “Facility and Equipment Confirmation”, which covers the following stages:
Verification Results

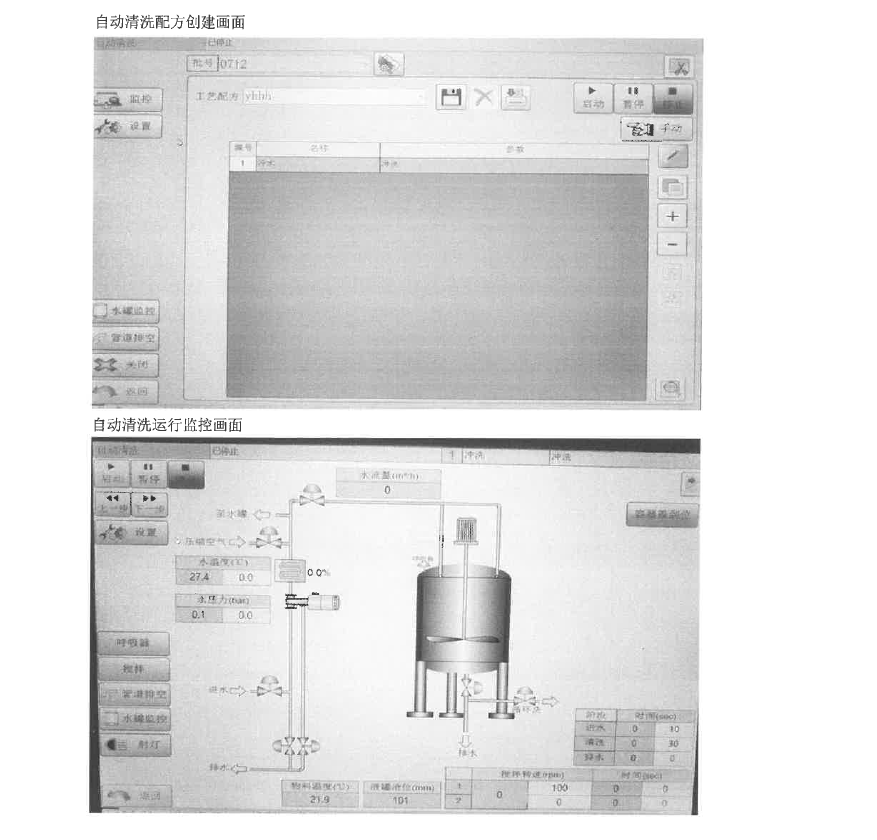
▲ Verify Document Diagram
Canaan Technology: A Reliable Verification Service Provider
Canaan Technology’s regulatory experts have extensive experience and have completed equipment validation and CSV validation for numerous pharmaceutical clients in China. They can provide efficient compliance consulting and validation services to enhance the performance and reliability of pharmaceutical equipment, ensuring its compliance and efficient operation in pharmaceutical environments.




Before any drug reaches a patient, it starts in a lab. That’s where formulas are tested, batches are checked, and quality is either confirmed or questioned. To do that work right, labs depend on the right equipment—tools that don’t just get the job done, but do it with precision. If you’re responsible for running or […]

Blister packaging is everywhere in pharma—from tablets to capsules to sample packs. It protects the product, extends shelf life, and improves patient safety. But for manufacturers, it’s more than just packaging—it’s a system built around speed, precision, and compliance. If you’re in pharma manufacturing or packaging procurement, here’s what you need to know about blister […]

If you’re deciding how to deliver a pharmaceutical or supplement product, the format you choose—liquid gels or tablets—will shape more than just how it looks. It affects how the product is made, how fast it’s absorbed, what kind of equipment you’ll need, and how the end user experiences it. Some actives work better in a […]